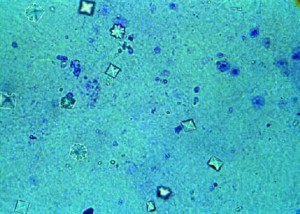
If you are new to the low oxalate diet, you may be wondering “What is oxalate?” All oxalate starts out as oxalic acid–a naturally-occurring strong acid that dissociates (ionizes) completely in water1. This complete dissociation allows oxalic acid to form strong bonds with many minerals, including calcium, iron, magnesium, potassium and sodium2. When oxalic acid binds with these minerals, it forms a salt which is then referred to as calcium oxalate, magnesium oxalate etc. If the salt easily dissolves in water (like table salt) it is called soluble oxalate. If it does not dissolve, but remains in a crystalline form, it is called insoluble oxalate2.
Both soluble and insoluble oxalate are commonly found in plants and to a much lesser extent in animals, including humans3. Although scientists aren’t sure why some plants contain a lot of oxalate, they have discovered that oxalate plays many roles in the plant’s ecology and biology.
Oxalate May Be Important For:
1.) Protecting the plant from insects and other foraging animals. For example, oxalate can cause burning, irritation, and stinging sensations in the mouths or skin of animals that eat it1,4,5
2.) Calcium regulation. For example, a plant might make more oxalate when intercellular calcium levels are too high so that the oxalate can bind with the calcium 1,4,5
3.) Heavy metal detoxification. For example, oxalate may bind with aluminum and help remove it from the plant1,4,5
4.) Tissue support. For example, calcium oxalate is often found in the cell wall of a plant–the structure that supports a plant and gives it shape (like bones support and give shape to many animals) 1,4
5.) Ion balance1,4
Oxalic acid is formed as part of the plants’ normal metabolic processes using numerous different biosynthesis pathways3,5 (we will explore a few of these in later articles). Researchers used to believe that oxalate was only a by-product in metabolism, but we now know that biosynthesis of oxalate is a carefully regulated process and that a plant can synthesize different sizes and shapes of calcium oxalate crystals depending on what function the oxalate will perform for the plant5. People also synthesize oxalate as part of our normal metabolic processes using the same biosynthesis pathways as plants5 (In people, we call this endogenous oxalate production). Most people and animals make negligible amounts of oxalate, but a few synthesize a lot, usually as the result of a genetic condition called primary hyperoxaluria (types I, II, or III) or because of a vitamin deficiency (often Vitamin B6 or thiamine).
Was this article helpful? What other types of articles would you enjoy seeing on Low Oxalate Info? Please let me know in the comments section below.
References:
{ 9 comments… read them below or add one }